Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yamano, Hidemasa; Kurisaka, Kenichi; Takano, Kazuya; Kikuchi, Shin; Kondo, Toshiki; Umeda, Ryota; Shirakura, Shota*
Dai-27-Kai Doryoku, Enerugi Gijutsu Shimpojiumu Koen Rombunshu (Internet), 5 Pages, 2023/09
This project studies investigation on safety design guideline and risk assessment technology for sodium-cooled fast reactor with the molten-salt heat storage system, development of evaluation method for heat transferring performance between sodium and molten-salt and improvement of the performance, and evaluation of chemical reaction characteristic between sodium and molten-salt and improvement of its safety. The project overview is presented in this report.
Yamano, Hidemasa; Kurisaka, Kenichi; Takano, Kazuya; Kikuchi, Shin; Kondo, Toshiki; Umeda, Ryota; Shirakura, Shota*; Hayashi, Masaaki*
Proceedings of 8th International Conference on New Energy and Future Energy Systems (NEFES 2023) (Internet), p.27 - 34, 2023/00
Times Cited Count:0This project studies investigation on safety design guideline and risk assessment technology for sodium-cooled fast reactor with the molten-salt heat storage system, development of evaluation method for heat transferring performance between sodium and molten-salt and improvement of the performance, and evaluation of chemical reaction characteristic between sodium and molten-salt and improvement of its safety. The project overview is presented in this report.
Uchino, Seiko*; Narita, Hirokazu*; Kita, Keisuke*; Suzuki, Hideya*; Matsumura, Tatsuro; Naganawa, Hirochika*; Sakaguchi, Koichi*; Oto, Keisuke*
Solvent Extraction Research and Development, Japan, 30(1), p.39 - 46, 2023/00
The extraction of trivalent rare earth ions (RE ) from HNO
) from HNO solution using a triamide amine, tris(N,N-di-2-ethylhexyl-ethylamide)amine (DEHTAA), was conducted, and the extraction mechanism was estimated from extraction behavior of HNO
solution using a triamide amine, tris(N,N-di-2-ethylhexyl-ethylamide)amine (DEHTAA), was conducted, and the extraction mechanism was estimated from extraction behavior of HNO and RE
and RE and the relationship between atomic number and extraction percentages (E%) for RE
and the relationship between atomic number and extraction percentages (E%) for RE . A DEHTAA molecule dominantly formed a DEHTAA HNO
. A DEHTAA molecule dominantly formed a DEHTAA HNO at 1.0 M HNO
at 1.0 M HNO and a DEHTAA(HNO
and a DEHTAA(HNO )
) at 6.0 M HNO
at 6.0 M HNO in the acid-equilibrated organic phase. This would provide the unique dependence of E% for the light RE
in the acid-equilibrated organic phase. This would provide the unique dependence of E% for the light RE on the HNO
on the HNO concentration, in which the E% value had a minimum and maximum at
concentration, in which the E% value had a minimum and maximum at  0.5 M and
0.5 M and  2 M HNO
2 M HNO , respectively. The results of the slope analyses for the distribution ratios for RE
, respectively. The results of the slope analyses for the distribution ratios for RE suggested that the dominant RE
suggested that the dominant RE complex was RE(NO
complex was RE(NO )
) DEHTAA(DEHTAA HNO
DEHTAA(DEHTAA HNO ) at 1.0 M HNO
) at 1.0 M HNO . The E% for RE
. The E% for RE decreased from La
decreased from La to Lu
to Lu at 1.0 M HNO
at 1.0 M HNO ; on the other hand, those increased from La
; on the other hand, those increased from La to Nd
to Nd at 0.25 M and from La
at 0.25 M and from La to Sm
to Sm and 6.0 M HNO
and 6.0 M HNO .
.
Ikeuchi, Hirotomo; Koyama, Shinichi; Osaka, Masahiko; Takano, Masahide; Nakamura, Satoshi; Onozawa, Atsushi; Sasaki, Shinji; Onishi, Takashi; Maeda, Koji; Kirishima, Akira*; et al.
JAEA-Technology 2022-021, 224 Pages, 2022/10
A set of technology, including acid dissolving, has to be established for the analysis of content of elements/nuclides in the fuel debris samples. In this project, a blind test was performed for the purpose of clarifying the current level of analytical accuracy and establishing the alternative methods in case that the insoluble residue remains. Overall composition of the simulated fuel debris (homogenized powder having a specific composition) were quantitatively determined in the four analytical institutions in Japan by using their own dissolving and analytical techniques. The merit and drawback for each technique were then evaluated, based on which a tentative flow of the analyses of fuel debris was constructed.
Hashikura, Yasuaki*; Ishijima, Yasuhiro; Nakahara, Masaumi; Sano, Yuichi; Ueno, Fumiyoshi; Abe, Hitoshi
Hozengaku, 19(3), p.95 - 102, 2020/10
A plutonium concentrator was selected, and constant load tensile tests with controlled applied potentials and electrochemical tests were conducted in nitric acid and sodium nitrate solutions. From the results, a map which shows the effect of nitric acid concentration to crack initiation potential was drawn. And, it was pointed out that not only the nitric acid but also the nitrate ion coordinated to the nitrate must be considered in evaluating the possibility of stress corrosion cracking.
Segawa, Tomoomi; Kawaguchi, Koichi; Ishii, Katsunori; Suzuki, Masahiro; Fukasawa, Tomonori*; Fukui, Kunihiro*
Funtai Kogakkai-Shi, 57(9), p.485 - 494, 2020/09
In the spent fuel reprocessing process, a mixed solution of uranyl nitrate and plutonium nitrate is converted into mixed oxide powder by the microwave heating. To evaluate the applicability to the industrial-scale and acquire the characteristics data of the microwave heating denitration of various metal nitrate aqueous solutions based on the knowledge studied in the development of laboratory-scale basic experiments, the microwave heating characteristics and metal oxide powder properties were investigated using cerium nitrate, cobalt nitrate and copper nitrate aqueous solutions. The progress rate of the denitration reaction was depended on the position, and the denitration reaction proceeded faster at the periphery than at the center. The morphologies of the synthesized products were porous and hard dry solid with cerium nitrate aqueous solution, foamed dry solid with cobalt nitrate aqueous solution, and powdery particles with copper nitrate aqueous solution. The denitration ratio and average particle size of the synthesized products increased in the order of the cerium nitrate aqueous solution, the cobalt nitrate aqueous solution, and the copper nitrate aqueous solution. The numerical simulations revealed that the periphery of the bottom surface of the metal nitrate aqueous solution was heated by microwaves. This results consistent with the experimental results in which the denitration reaction started from the periphery of the metal nitrate aqueous solution.
Kato, Chiaki
Comprehensive Nuclear Materials, 2nd Edition, Vol.4, p.528 - 563, 2020/08
In spent fuel reprocessing plants, various nitric media are encountered throughout the PUREX process, used in the separation of fission products, uranium, and plutonium. The PUREX process is thus highly corrosive as it takes place at high temperatures under high concentrations of nitric acid solution containing oxidizing metal ions from spent fuel. In this review, the unique chemical properties of nitric acid are first described. Secondly, the process of oxidizing power generation in boiling nitric acid under heat transfer is described using the redox potential and a thermodynamic model of boiling nitric acid. Finally, the corrosion behavior and corrosion acceleration mechanism specific to the reprocessing environments are described from the perspective of solution chemistry.
Abe, Toru*; Hirano, Fumio; Mihara, Morihiro; Honda, Akira
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 27(1), p.3 - 11, 2020/06
Degradation of TRU waste in a geological disposal facility may cause the formation of a nitrate plume. A Nitrate Evolution model due to mineral reactions, microbial activity, and metal corrosiON (NEON) has therefore been developed to evaluate the safety case for geological disposal of TRU waste. Small scale laboratory experiments can be reproduced satisfactorily, however, it is necessary to demonstrate the applicability of the NEON model on scales relevant to the geological disposal of TRU waste. In the current study, an industrial analogue of a nitrate plume from the pollution of groundwater from nitrate fertilizers used on Ikuchi Island, Japan was selected to test the applicability of the NEON model. Concentration profiles of nitrate ions in the groundwater were successfully reproduced over the hundreds of meters scale demonstrating the applicability of the NEON model in evaluating the chemical behavior of a nitrate plume derived from the geological disposal of TRU waste.
Sasaki, Yuji; Ban, Yasutoshi; Morita, Keisuke; Matsumiya, Masahiko*; Ono, Ryoma*; Shiroishi, Hidenobu*
Solvent Extraction Research and Development, Japan, 27(1), p.63 - 67, 2020/00
Times Cited Count:6 Percentile:31.74(Chemistry, Multidisciplinary)Mutual separation technique of Dy and Nd in Nd magnet is studied. Dy is more valuable than Nd, then Dy might be isolated and reused. Lanthanide elements can be extracted thoroughly by diglycolamide (DGA) extractants, we use this reagent for the recovery and isolation of Dy. Tetradodecyl-DGA (TDdDGA) has relatively high separation factors(SF) between Dy and Nd (SF=17-18) in HNO extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO
extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO , 92% Dy can be recovered with 0.7% co-extraction of Nd.
, 92% Dy can be recovered with 0.7% co-extraction of Nd.
Hayashi, Hirokazu; Chiba, Rikiya*
Progress in Nuclear Science and Technology (Internet), 5, p.196 - 199, 2018/11
Uranium-free nitride fuel has been chosen as the first candidate for transmutation of long-lived minor actinides (MA: Np, Am, Cm) using sub-critical accelerator-driven system (ADS) under the double strata fuel cycle concept by Japan Atomic Energy Agency (JAEA). Dissolution behavior of ZrN-based nitrides in nitric acid is examined using lanthanides as surrogate materials of TRU elements. Chemical analysis of the ZrN-based lanthanide nitrides dissolved in nitric acid is also carried out.
Sano, Yuichi; Ambai, Hiromu; Takahatake, Yoko
Aichi Shinkurotoron Hikari Senta 2017-Nendo Kokyoto Riyo Seika Hokokusho (Internet), 1 Pages, 2018/00
In order to elucidate the mechanism of corrosion in the reprocessing process and propose a method for suppressing corrosion, the effect of coexisting substances on the chemical form of Ru in nitric acid solution containing seawater components was evaluated. The result of XAFS measurement for Ru showed the structural change around a Ru atom due to the interaction with chloride ion, which will suppress the corrosion promoting action of Ru in nitric acid solution.
Tanno, Takashi; Takeuchi, Masayuki; Otsuka, Satoshi; Kaito, Takeji
Journal of Nuclear Materials, 494, p.219 - 226, 2017/10
Times Cited Count:17 Percentile:85.35(Materials Science, Multidisciplinary)Oxide dispersion strengthened (ODS) steel cladding tubes have been developed for fast reactors. 9 chromium ODS and 11Cr-ODS tempered martensitic steels are prioritized for the candidate material in research being carried out at JAEA. In this work, fundamental immersion tests and electro-chemical tests of 9 to 12Cr-ODS steels were systematically conducted in various nitric acid solutions at 95 C. The corrosion rate exponentially decreased with effective solute chromium concentration (Cr
C. The corrosion rate exponentially decreased with effective solute chromium concentration (Cr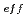 ) and nitric acid concentration. Addition of oxidizing ions also suppressed the corrosion rate. According to polarization curves and surface observations in this work, the combination of low Cr
) and nitric acid concentration. Addition of oxidizing ions also suppressed the corrosion rate. According to polarization curves and surface observations in this work, the combination of low Cr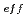 and dilute nitric acid could not prevent the active dissolution at the beginning of immersion, and the corrosion rate was high. In comparison, higher Cr
and dilute nitric acid could not prevent the active dissolution at the beginning of immersion, and the corrosion rate was high. In comparison, higher Cr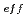 , concentrated nitric acid and addition of oxidizing ions helped to prevent the active dissolution, and suppressed the corrosion rate.
, concentrated nitric acid and addition of oxidizing ions helped to prevent the active dissolution, and suppressed the corrosion rate.
Segawa, Tomoomi; Fukasawa, Tomonori*; Huang, A.-N.*; Yamada, Yoshikazu; Suzuki, Masahiro; Fukui, Kunihiro*
Chemical Engineering Science, 153, p.108 - 116, 2016/10
Times Cited Count:7 Percentile:26.49(Engineering, Chemical)The influence of the heating method and rate on the morphology of CuO powders synthesized from Cu(NO )
)
 3H
3H O aqueous solutions by denitration was investigated. The median diameter of the obtained powder was found to decrease as the heating rate increased, independent of the heating method. The microwave heating method remarkably reduced the particle size and enhanced the irregularity and disorder of the shape and surface of the particles, which were found to be more widely distributed. In contrast, the microwave hybrid heating method yielded the most spherical particles with the smoothest surface. It was also found that this heating method sharpened the particle size distribution and had higher energy efficiency than the MW method. Numerical simulations also indicated a difference in the energy efficiency between these two methods. The simulations also revealed that the hybrid method could heat the whole reactor more uniformly with a lower microwave output.
O aqueous solutions by denitration was investigated. The median diameter of the obtained powder was found to decrease as the heating rate increased, independent of the heating method. The microwave heating method remarkably reduced the particle size and enhanced the irregularity and disorder of the shape and surface of the particles, which were found to be more widely distributed. In contrast, the microwave hybrid heating method yielded the most spherical particles with the smoothest surface. It was also found that this heating method sharpened the particle size distribution and had higher energy efficiency than the MW method. Numerical simulations also indicated a difference in the energy efficiency between these two methods. The simulations also revealed that the hybrid method could heat the whole reactor more uniformly with a lower microwave output.
Ueno, Fumiyoshi; Irisawa, Eriko; Kato, Chiaki; Igarashi, Takahiro; Yamamoto, Masahiro; Abe, Hitoshi
Proceedings of European Corrosion Congress 2016 (EUROCORR 2016) (USB Flash Drive), 7 Pages, 2016/09
In this study, we focused on the effect of the boiling of nitric acid solution on the corrosion of a stainless steel-made concentrator in reduced pressure in fuel reprocessing plant. In order to perform the simulation test in a non-radioactive condition, nitric acid solution with the addition of vanadium as an oxidizing metal ion were used. Corrosion tests were carried out under the conditions of boiling at reduced pressure, and of non-boiling at normal pressure and several temperatures. As a result, corrosion was accelerated by the solution boiling while it was not by non-boiling at the same temperature. It was found also that the temperature dependence of corrosion rate is the same in the both conditions of boiling and non-boiling. The corrosion accelerating effect will be discussed on the basis of the reaction among nitric acid, NOx and vanadium, etc.
Irisawa, Eriko; Ueno, Fumiyoshi; Kato, Chiaki; Abe, Hitoshi
Zairyo To Kankyo, 65(4), p.134 - 137, 2016/04
In order to investigate the effect of boiling under reduced pressure on corrosion of stainless steel in the nitric acid solution, the corrosion tests simulating the high-level radioactive liquid waste evaporator were performed. The results of immersion tests of stainless steels in the solution with and without boiling showed that the corrosion rates in boiling solution were larger than those in not boiling solution in case of same temperature of solution. Moreover, the cathode polarization curves showed that the corrosion potential of stainless steel in boiling solutions were shifted nobler, and the current intensity became larger than that in not boiling solutions. According to these results, it can be concluded that boiling of solution under reduced pressure accelerate the corrosion rates.
 -tetraoctyl-thiodiglycolamide
-tetraoctyl-thiodiglycolamideSasaki, Yuji; Suzuki, Tomoya; Morita, Keisuke; Yoshizuka, Kazuharu*
Hydrometallurgy, 159, p.107 - 109, 2016/01
Times Cited Count:5 Percentile:27.46(Metallurgy & Metallurgical Engineering)The novel tridentate extractant including soft donor has developed and examined. The extractant, tetraoctyl-thiodiglycolamide (S-DGA), is analogous structure to tetraoctyl-diglycolamide (TODGA), but with sulfur donor instead of ether oxygen of TODGA. S-DGA has unique property to extract silver from acidic solutions to n-dodecane with relatively high D values. We investigated the extraction behavior of Ag in various acids, HNO , H
, H SO
SO , and HClO
, and HClO .
.
Segawa, Tomoomi; Kawaguchi, Koichi; Ishii, Katsunori; Suzuki, Masahiro; Arimitsu, Naoki*; Yoshida, Hideto*; Fukui, Kunihiro*
Advanced Powder Technology, 26(3), p.983 - 990, 2015/05
Times Cited Count:8 Percentile:27.86(Engineering, Chemical)Denitration of the aqueous solution of nickel nitrate hexahydrate (Ni(NO )
)
 6H
6H O) by a microwave heating method was investigated. Since Ni(NO
O) by a microwave heating method was investigated. Since Ni(NO )
)
 6H
6H O aqueous solution cannot be heated to over 300
O aqueous solution cannot be heated to over 300  C by microwave irradiation owing to the low microwave absorptivity of its intermediate, NiO could not previously be obtained by microwave heating. We propose a novel NiO synthesis method that uses microwave heating without the risk of chemical contamination. A NiO powder reagent was added to the solution as a microwave acceptor. The denitration efficiency to NiO could be improved by an adiabator around the reactor to increase the temperature homogeneity in the reactor. Numerical simulations also reveal that the use of the adiabator results in remarkable changes in the electromagnetic field distribution in the reactor, temperature inhomogeneity decreases.
C by microwave irradiation owing to the low microwave absorptivity of its intermediate, NiO could not previously be obtained by microwave heating. We propose a novel NiO synthesis method that uses microwave heating without the risk of chemical contamination. A NiO powder reagent was added to the solution as a microwave acceptor. The denitration efficiency to NiO could be improved by an adiabator around the reactor to increase the temperature homogeneity in the reactor. Numerical simulations also reveal that the use of the adiabator results in remarkable changes in the electromagnetic field distribution in the reactor, temperature inhomogeneity decreases.
Nakamura, Yasuo; Nakatani, Takayoshi
JAEA-Technology 2014-048, 18 Pages, 2015/03
Sodium nitrate is included bituminized waste generating from the reprocessing plant of spent fuel which is disposed of in sub-surface disposal facility. Because the sodium nitrate is soluble material in surface water, it is a concern impact on surface water. Such as non-radioactive materials are not strictly regulated by "the Law for the Regulations of Nuclear Source Material, Nuclear Fuel Material and Reactors", but should be considered by related laws and regulations according to former basic policy. Because it is regulated as nitrate nitrogen by "The Basic Environment Law", the valuation of the environmental impact on general sub-surface disposal system was carried out. As the results, the concentration of nitrate nitrogen in river water whose annual quantity of water is rather than 1 10
10 m
m /y is below the regulated value at the small scale surface waters as evaluation point.
/y is below the regulated value at the small scale surface waters as evaluation point.
Fukui, Kunihiro*; Igawa, Yusuke*; Arimitsu, Naoki*; Suzuki, Masahiro; Segawa, Tomoomi; Fujii, Kanichi*; Yamamoto, Tetsuya*; Yoshida, Hideto*
Chemical Engineering Journal, 211-212, p.1 - 8, 2012/11
Times Cited Count:13 Percentile:41.13(Engineering, Environmental)The process for synthesizing metallic oxide powders by the microwave denitration method was investigated using hexahydrated nickel nitrate and trihydrated copper nitrate aqueous solutions, and the electrical field and the temperature distributions in the reactor were numerically simulated. Although CuO powder can be obtained from a trihydrated copper nitrate aqueous solution by the microwave denitration method, a hexahydrated nickel nitrate aqueous solution cannot be heated up to over 270  C by microwave irradiation. It was also found that the reaction routes for microwave heating are the same as those for conventional external heating. This finding indicates that the success of producing oxide particles by microwave denitration depends not only on the microwave absorptivity of the intermediate and the metallic oxide, but also on the temperature difference.
C by microwave irradiation. It was also found that the reaction routes for microwave heating are the same as those for conventional external heating. This finding indicates that the success of producing oxide particles by microwave denitration depends not only on the microwave absorptivity of the intermediate and the metallic oxide, but also on the temperature difference.
Saito, Shigeru; Sasa, Toshinobu; Umeno, Makoto*; Kurata, Yuji; Kikuchi, Kenji; Futakawa, Masatoshi
JAERI-Tech 2004-074, 41 Pages, 2004/12
The accelerator driven system (ADS) is proposed to transmute minor actinides (MA) in high-level waste from spent fuels of nuclear power reactors. Liquid Pb-Bi alloy is a candidate material for spallation target and coolant of ADS. Pb-Bi cleaning technology is required to reduce radiation exposure during maintenance service and to decontaminate replaced components. In this study, three cleaning methods were tested; silicon oil cleaning at 170 C, mixture of acetic acid and nitric acid cleaning. Specimens were prepared by immersion in melted Pb-Bi. After silicon oil tests, most of Pb-Bi remained on the surface of the specimens. It was found that blushing was needed to remove Pb-Bi effectively. On the other hands, Pb-Bi was easily dissolved and almost removed in the mixed acid and nitric acid. Silicon oil cleaning did not affect on base metals. The surface of base metals was slightly blacked after mixed acid cleaning. F82H base metals were corroded by nitric acid.
C, mixture of acetic acid and nitric acid cleaning. Specimens were prepared by immersion in melted Pb-Bi. After silicon oil tests, most of Pb-Bi remained on the surface of the specimens. It was found that blushing was needed to remove Pb-Bi effectively. On the other hands, Pb-Bi was easily dissolved and almost removed in the mixed acid and nitric acid. Silicon oil cleaning did not affect on base metals. The surface of base metals was slightly blacked after mixed acid cleaning. F82H base metals were corroded by nitric acid.